
All About Nitrogen
Nitrogen: crucial to the life of plants and organisms and a necessary component of proteins. Beyond biology, it plays a critical role in countless scientific and industrial applications. We've gathered everything you need to know about this fascinating element: its properties, uses, and what makes it indispensable in research and technology.

Nitrogen and life on Earth
Why is nitrogen so important to us, you ask? Nitrogen is a universal building block of life due to it being a key component of amino acids, proteins, nucleic acids (DNA and RNA), and chlorophyll in plants. These molecules are essential for the growth, reproduction, and metabolic processes of living organisms, from plants to animals. Without nitrogen, life as we know it cannot exist.

Why is nitrogen important to plants?
Nitrogen is a critical element for plants because it forms the backbone of chlorophyll, the green pigment responsible for capturing light energy during photosynthesis in plants (as well as algae and some bacteria). Chlorophyll molecules contain nitrogen atoms within their structure, enabling them to absorb sunlight and convert it into chemical energy.
Photosynthetis is the biological process by which green plants, algae, and bacteria convert light energy into chemical energy, during which oxygen gets released. More chlorophyll means better light absorption, supporting efficient conversion at an optimal rate.
Beyond chlorophyll, nitrogen is a key component of amino acids and enzymes that power the biochemical reactions of photosynthesis, such as synthesizing glucose from carbon dioxide and water.
Without sufficient nitrogen, plants cannot produce enough chlorophyll or maintain enzyme activity, leading to:
- Reduced photosynthetic efficiency,
- Slower growth,
- Pale or yellow leaves (called chlorosis).
Essentially, nitrogen fuels the entire photosynthetic machinery, transforming light energy into the sugars that sustain plant life.

How do plants get nitrogen?
Most plants absorb nitrogen in the form of nitrate (NO₃⁻) and ammonium (NH₄⁺) ions from soil water. These come from the natural breakdown of organic matter, as well as fertilisers. Certain bacteria living in root nodules also convert atmospheric nitrogen to ammonia, which the plants can use.
Soil microbes decomposing organic matter also releases nitrogen as ammonium. While plants can absorb both ammonium and nitrate, nitrate is generally easier for roots to take up, making it the preferred form in most soils.
Key Nitrogen Sources for Plants
- Fertilizers: A source of readily available nitrate and ammonium.
- Organic matter: Provided from decomposed plant and animal matter, releases ammonium.
- Nitrogen-fixing bacteria: Convert atmospheric nitrogen into usable forms.
- Soil bicrobes: Assist in the breakdown of down organic matter and release ammonium.

How does nitrogen become usable?
Although nitrogen makes up about 78% of Earth’s atmosphere, most of it exists as atmospheric nitrogen (N₂), which is highly stable and unusable by most living organisms. This chemical inertness means that, despite its abundance, biologically available nitrogen (one that living organisms can readily absorb and use) is scarce in many ecosystems.
For nitrogen to become biologically available, it must be converted into reactive forms such as:
- Ammonium (NH₄⁺)
- Nitrate (NO₃⁻)
- Nitrite (NO₂⁻)
- Organic nitrogen compounds (like amino acids).
The nitrogen cycle explained
The way in which nitrogen moves through different chemical forms as it circulates among the atmosphere, land, and marine ecosystems is called the nitrogen cycle. These transformations occur through both physical and biological mechanisms.
Click on the image to explore the cycle:
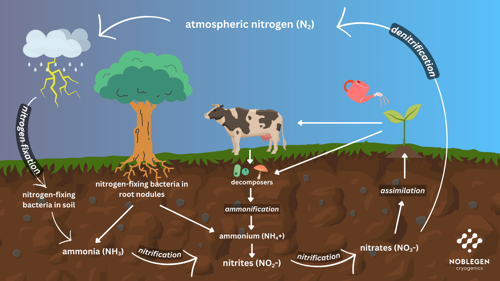
1. Nitrogen Fixation
- At the start of the cycle, atmospheric nitrogen (N₂) is converted into biologically usable forms (ammonia/NH₃ or ammonium/NH₄⁺).
- This is achieved by:
- Nitrogen-fixing bacteria in soil or root nodules of legumes.
- Lightning and industrial processes (Haber-Bosch).
2. Nitrification
- Soil bacteria convert ammonium (NH₄⁺) into nitrite (NO₂⁻) and then nitrate (NO₃⁻).
- Nitrate is the main form plants absorb.
3. Assimilation
- Assimilation is when plants take up nitrate or ammonium and use it to build proteins and nucleic acids.
- Animals get nitrogen by eating plants or other animals.
4. Ammonification
- When plants and animals die or excrete waste, decomposers break down organic nitrogen into ammonium.
5. Denitrification
- Bacteria, such as pseudomonas convert nitrate back into nitrogen gas (N₂), releasing it into the atmosphere.
- This closes the cycle.

Industrial and agricultural uses of nitrogen
Agricultural Use
Nitrogen is a fundamental nutrient for plant growth and the primary component of most fertilizers. It helps to improve crop yields, supports healthy soil, and ensures sustainable food production.
Industrial Use
In industry, nitrogen is indispensable for a wide range of processes. It is used to produce ammonia through the Haber-Bosch process, which serves as the foundation for fertilizers and several other chemicals. Nitrogen also acts as an inert gas in manufacturing, preventing oxidation during metal production, electronics assembly, and pharmaceutical processing. In its liquid form, nitrogen is a cryogen (a liquefied gas with a boiling point of -196°C/-320°F), used in applications from extreme cold temperature storage to cooling scientific instruments and preserving various materials.
Environmental Impact
While nitrogen is essential, its use must be managed responsibly. Excessive application in agriculture can lead to water pollution and greenhouse gas emissions. Modern practices focus on optimizing nitrogen use to balance productivity with environmental sustainability.
5 key characteristics of nitrogen
Atomic Mass
Nitrogen has an atomic mass of approximately 14.01 u and an atomic number of 7, making it a relatively light element essential for life.
Abundance
It is the most abundant gas in Earth’s atmosphere, comprising about 78% of the air, though most of it exists as inert N₂ molecules.
Chemical Reactivity
Atmospheric nitrogen (N₂) is highly stable due to its triple bond, making it difficult for most organisms to use directly. It requires nitrogen fixation to become biologically available.
State
At standard temperature and pressure, nitrogen is a colorless, odorless gas, but it can transition to liquid form under cryogenic conditions.
Boiling Point
Liquid nitrogen has a boiling point of approximately –196°C/–321°F. It is widely used for cooling, preservation, and testing in industries such as pharmaceutical, electronics, and research, where ultra-low temperatures are critical.

How many bonds can nitrogen form?
Nitrogen can form up to three covalent bonds, and that’s the key to its incredible versatility. Why three? Because nitrogen has five electrons in its outer shell and atoms are most stable when they have eight (this is called the octet rule). Nitrogen is highly adaptable - it can bond not only with hydrogen (as in ammonia, NH₃) but also with carbon and other elements to form countless compounds.
This simple fact explains a lot:
- It’s why nitrogen forms ammonia (NH₃), a building block for fertilizers.
- It’s why nitrogen gas (N₂) is so stable—two nitrogen atoms share three pairs of electrons, creating a strong triple bond that makes up most of our atmosphere.
- And it’s why nitrogen can even stretch to four bonds in special cases like ammonium (NH₄⁺), by giving up its lone pair and carrying a positive charge.
In short, nitrogen’s bonding ability shapes everything from plant growth to industrial chemistry, and even the molecules of life.

Is nitrogen heavier than air?
No, air and nitrogen have a very similar molecular weight and both behave very similarly under normal conditions. This is because air is made up of a mixture of gases with approximately 78% nitrogen, with the different types of gases being constantly mixed due to motion and wind. This can change if nitrogen is cooled down: in this case, it will become denser and settle on the ground (the same way cold air is denser than warm air).

Nitrogen fun facts you should know
Nitrogen in Space
Nitrogen isn’t just on Earth; it’s found on Titan (Saturn’s moon), where it makes up most of the atmosphere, similar to Earth. It's also present on moons like Triton where nitrogen-rich ice is found on the surface, as well as the soil of Mars. See the connection between our nitrogen generators and their space-inspired names now?
The Origin of Life
Nitrogen compounds are thought to have played a key role in the origin of life, forming amino acids and nucleotides in early Earth conditions.
Biological Adaptations
Some plants, like legumes, form symbiotic relationships with nitrogen-fixing bacteria, creating root nodules that act as natural fertilizer factories.
Nitrogen and Climate Impact
Nitrous oxide (N₂O), a byproduct of the nitrogen cycle, is a potent greenhouse gas—about 300 times more effective at trapping heat than CO₂. This links nitrogen management directly to climate change.

What is a nitrogen generator?
Nitrogen generators separate nitrogen molecules from ambient air to produce a pure, on-demand supply of nitrogen for scientific or industrial use.
Nitrogen generation systems use membrane separation or PSA (Pressure Swing Adsorption) technology to isolate nitrogen from other gases, such as oxygen, carbon dioxide, and water vapor. Following separation, the output is a steady supply of pure nitrogen gas that's ready to be used or stored. Liquid nitrogen generators take this process a step further by cooling the gas to cryogenic temperatures using a cryocooler, and collect the condensed liquid nitrogen in a vacuum-insulated internal storage tank.
Noblegen Liquid Nitrogen Generators
Explore our range of generators supporting applications with liquid nitrogen for cryogenics, academic research, animal husbandry, IVF, dermatology and beyond.




